 |
 |
|
|
|
|
Student Work #2Task: At this station, you will be establishing the conditions under which a new experimental drug will be tested.Background The drug ALAMAIN has been developed by the Gentronic Drug Company to lower blood pressure in people whose blood pressure is too high. The drug has been thoroughly tested on animals with positive results. The Gentronic Drug Company feels it is now time for the drug to be tested on humans, and have contacted the Human Improvement Laboratory to do the testing. Directions As chief research scientist at the Human Improvement Laboratory (HIL) you have been assigned the task of developing the human testing program for the new high blood pressure drug Alamain. You and your assistants are to confer on the experimental design of this testing program, and to write a report outlining the program. The report is to be submitted to the chairperson of the HIL Drug-Testing Committee for approval. Complete the following sections as you would include them on your report.

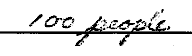
B. Using complete sentences explain your answer. 
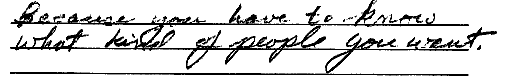


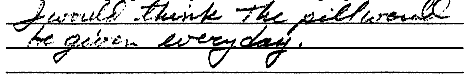

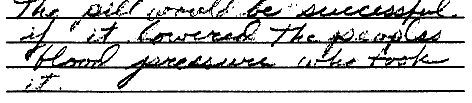
ScoringCircle the student's score for each question. Add points for each question and write the total score at the bottom of the scoring form. 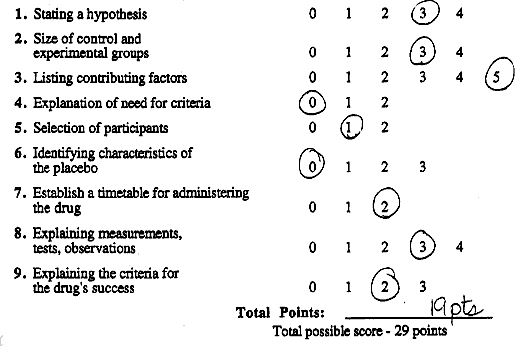
Previous Student Work | Next Student Work
|
