Contributed by: Connecticut Academic Performance Test (CAPT)
You will be investigating a problem related to acid rain. During this
activity, you will work with a partner (or possibly two partners).
However, you must keep your own individual lab notes because after
you finish you will work independently to write a report about your
investigation.
The Problem
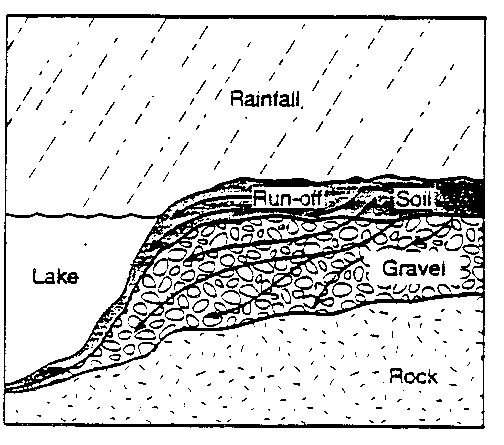
Acid rain refers to rain, snow or other precipitation with a pH below
5.6. In extreme cases, acid rain can have a pH as low as 2.0! Many
lakes in the Northeastern United States appear to be suffering harmful
effects from acid rain. Although they often look crystal clear, these
lakes have significant decreases in their number of fish and other
life forms.
Yet other lakes in the same region seem healthy. The reason may have
to do with the rocks and soil that surround the lakes. Some rain falls
directly into rivers and lakes, but much of it hits the ground first
and then flows or seeps into the bodies of water (see the diagram
below). When acid rain flows through soil and gravel that have a higher
pH than the rain, the pH of the rain may change. The rate at which
the rain percolates or seeps through the soil and gravel is another
important factor that affects the neutralization of the acid. If it
seeps through too quickly, there may not be time for a noticeable
change in pH to occur.
Your Task
You and your partner will design and conduct experiments to determine
which earth material (sand, potting soil or limestone) or combination
of earth materials will best reduce the acidity of "acid rain." You
will use a vinegar-and-water solution as substitute for acid rain.
You will investigate the problem by studying the percolation rate
(the rate at which water seeps through a material) and neutralizing
ability (ability of materials to reduce the acidity of acids) of various
earth materials.
You have been provided with the following materials and equipment:
Sand (1/2 cupful)
Potting soil (1/2 cupful)
Crushed limestone (1/2 cupful)
Vinegar (200 mL)
Tap water
pH test strips (10) and pH color chart
Access to a balance
Access to a clock or a watch with a second hand
Access to a calculator
Paper towels for clean-up
Safety equipment (including goggles and aprons) |
Ruler
Plastic spoon
Paper cups (8)
Square bandages (4)
Wooden sticks (6)
Graduated cylinder
Large beaker |
|
Steps to Follow
- In your own words, state the problem you are going to
investigate, and write your statement of the problem on the page
provided.
- Design one or more experiments to solve the problem.
Describe your experimental designs on the page provided. Show
your designs to your teacher before you begin your experiments.
(Hint: There are several ways to investigate this problem. The
illustration below may give you one idea for designing your experiments.)
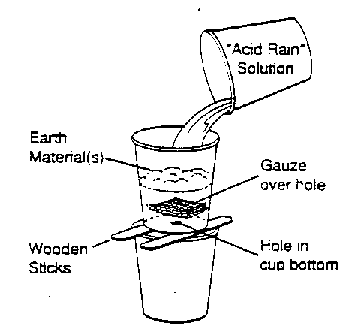
- Mix the 200 mL of vinegar with 100 mL of water in the
large beaker to create an "acid rain" solution. Use the plastic
spoon to stir the vinegar and water, and then use a pH test strip
to determine the acidity of the solution.
- After receiving approval from your teacher, work with
your partner to carry out your experiments. Your teacher's approval
does not necessarily mean that your teacher thinks your experiments
are well designed. It simply means that in your teacher's judgment
your experiments are not dangerous or likely to cause an unnecessary
mess.
- While conducting your experiments, take notes on the
pages provided. Space is also provided for charts, tables or graphs.
Your notes will not be scored, but they will be helpful
to you later as you work independently to write about your experiment
and results. You must keep your own notes because you will
not work with your partner when you write your report.
Later, you will work independently to write about your investigation
in the form of a report to a planning commission that is trying
to decide which earth material should be used around a local lake
in order to reduce the effects of acid rain. Take a few minutes
to find out what you should include in your report.
When you have finished your experiment, your teacher will give
you instructions for cleanup procedures, including proper disposal
of all materials.
(Students are provided with 4 blank pages for their notes)
Directions for Writing Your Report
You will now summarize your experiments and results in the form of
a report to a planning commission that is trying to decide which earth
material should be used around a local lake in order to reduce the
effects of acid rain. You may use the lab notes you took while working
with your partner. You may wish to write a first draft of your report
on scratch paper, but your final copy should be written on the pages
provided. Space for charts, tables or graphs is provided.
Your report should include:
- a clear statement of the problem you investigated;
- a description of the experiments you carried out;
- the results of your experiments (including data presented in
the form of charts, tables or graphs);
- your conclusions from the experiments; and
- comments about how valid you think your conclusions are. (In
other words, how much confidence do you have that your results
are accurate? What errors may have affected your results?)
(Students are provided with 4 blank pages for their report)
CAPT Experimentation Questions
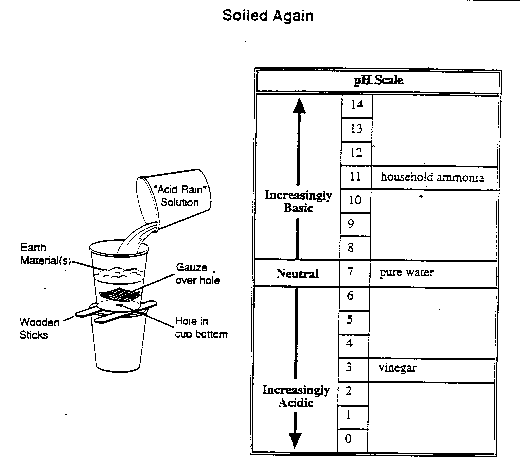
A group of students prepared a vinegar-and-water solution to simulate
acid rain. After determining its pH, they poured the solution through
cups that contained various earth materials (sand, potting soil and
crushed limestone). The students measured the amount of solution that
percolated (passed) through a hole in the bottom of each cup in a
set amount of time, and they tested the pH of the drippings.
The results of the group's experiment are shown in the following table.
| Earth Materials |
pH of "acid rain" before percolation |
Amount of "acid rain" percolated in 3 minutes |
pH of percolated "acid rain" |
| sand |
3.0 |
30ml |
3.5 |
| potting soil |
3.0 |
20ml |
3.5 |
| crushed limestone |
3.0 |
90ml |
5.0 |
| all three Earth materials |
3.0 |
50ml |
5.5 |
- What is one problem that this group is investigating? State
the problem in your own words.
- What are the variables that need to be controlled in this experiment?
Explain why it is important to control them.
- Do you have enough information to replicate this group's experiment?
If you think you do, tell what information you have. If you think
you do not, tell what other information you would need.
- The group concluded that sand and potting soil have the same
ability to neutralize acidity because in each case the pH went
from 3.0 to 3.5. Based on this group's experiment and results,
do you think the group's conclusion is valid? Explain why or why
not.
|
|




