|
Contributed by: Kentucky Department of Education (KDE)
PERFORMANCE TASK STUDENT RESPONSE FORM
TASK: S2 - Water Pollution
Grade 8
Student Name: _______________________________________________
School Name: _______________________________________________
GENERAL INSTRUCTIONS:
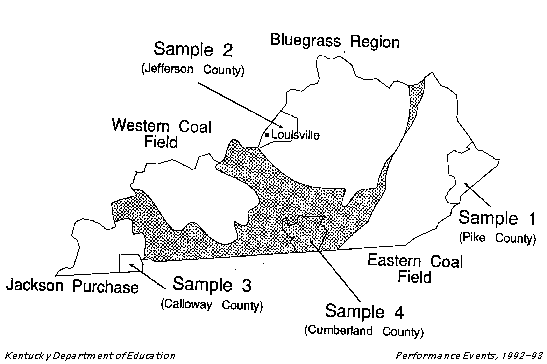
You are a scientist who has collected four samples of rain water
from different parts of Kentucky, as shown by the map on page 2
of this booklet. Your task will be to determine the pH of the samples
and then to neutralize them. You will be working as a group for
the first 25 minutes of this activity.
MATERIALS:
- dropping bottles with solutions (samples 1-4)
- universal indicator
- pH paper
- map of Kentucky (page 2)
- acid solution for neutralization
- basic solution for neutralization
- stirring sticks
- safety glasses
- paper towels
INSTRUCTIONS:
- Place 20 drops of each sampling into the mixing tray in the
corresponding depression (sample 1 in depression 1, etc.)
- Using the pH paper and the pH color scale, determine the pH
of each of the four samples. Record this information in the table
at the bottom of this page. If any sample changes color on contact
with pH paper, absorb the sample with a paper towel and replace
that sample.
- Place 1 drop of the universal indicator in each depression containing
a sample.
- Slowly (one drop at a time), place drops of the appropriate
neutralizer into each sample (acid into a base or vice versa).
Stir the sample after adding each drop. Continue this until a
color change occurs and remains after stirring. Record the number
of drops needed to neutralize (cause a color change) in each sample.
Record all data in the table below.
| SAMPLE |
pH |
COLOR (FROM-TO) |
#OF DROPS TO NEUTRALIZE |
| 1 |
|
|
|
| 2 |
|
|
|
| 3 |
|
|
|
| 4 |
|
|
|
NOTE:
A substance with pH less than 7 is acidic, equal to 7 neutral, greater
than 7 basic. The strongest acid has a pH equal to 0, and the strongest
base has pH equal to 14.
You will have approximately 20 minutes to answer questions
5-7 on your own.
- Consider the data you have collected and the information provided
on the map on page 4, decide which sample shows the most serious
environmental problem in its region. Give possible causes for
the problems you identify.
__________________________________________________________________________
__________________________________________________________________________
__________________________________________________________________________
__________________________________________________________________________
__________________________________________________________________________
- Predict any negative effects which might be associated with
rain water having a pH reading you identified as problematic in
question 1. What evidence that these effects have occurred would
you look for?
__________________________________________________________________________
__________________________________________________________________________
__________________________________________________________________________
__________________________________________________________________________
__________________________________________________________________________
- As a scientist and a citizen in a region showing some of these
environmental problems, what steps would you take to deal with
the problems?
__________________________________________________________________________
__________________________________________________________________________
__________________________________________________________________________
__________________________________________________________________________
__________________________________________________________________________
- Discuss procedures or other variables which may have affected
the data you obtained in your experiment.
__________________________________________________________________________
__________________________________________________________________________
__________________________________________________________________________
__________________________________________________________________________
__________________________________________________________________________
Kentucky Department of Education
Performance Events 1992-93
|


