|
Contributed by: Council of Chief State School Officers (CCSSO)
Item Description:
This investigation is intended to show the student the relationship
between the temperature and reaction rate of a mixture. The
student is told that the reaction rate, defined as the amount
of time for a given number of atoms and molecules to react,
depends on the average speed of the molecules which depends
on the temperature.
The student adds two reactants to a uniform amount of water
at three different temperatures (all hot, 3 parts hot: 1 part
cold, and 1 part hot: 1 part cold). The student stirs the mixture
and observes its color, recording the amount of time it takes
for the reaction to occur (as indicated by color change from
purple to yellow).
Question 1 asks the student to graph the temperature of the
mixture against the time the reaction occurred for each case.
A good answer will consist of a properly labeled graph. Each
axis should be labeled either "time" or "temperature" and the
axes should be marked with a scale approximate for the data
obtained. The three graphed points should be connected with
a straight line. The line does not have to pass through each
point but should come as close to each point as possible. The
graph should fill at least 1/2 of the graph paper provided.
Temperature should be on the "y" axis and time on the "x" axis.
An example follows under Criterion 2.
Question 2 asks the student to explain how the relationship
between temperature and reaction rate can be applied to everyday
life and give an example to support the explanation. A good
response should state the relationship between temperature and
reaction rate and describe a specific situation where temperature
is related to reaction rate in an everyday experience. This
explanation should be scientifically correct. In addition, the
student's explanation should be supported by a specific example.
The Item:
Question #1: On the sheet of graph paper below, plot the temperature
(degrees Celsius) of the mixture versus the time (seconds, or
sec) when the mixture began to turn yellow. Draw a smooth line
that passes near (but not necessarily through) all the data
points.
Question #2: On the basis of your conclusion in Question 2,
explain how the relationship between temperature and reaction
rate is used in everyday life. Give an example to support your
explanation.
Comments:
In the data table it is more appropriate, given the length of
the experiment, to calculate the reaction times in minutes instead
of seconds. Therefore, the graph may be labeled in minutes or
seconds. Also, due to the length of this experiment, many students
did not complete the data collection. Criterion 1 takes this
situation into account. Finally, there is a misprint in Question
2.
"On the basis of your conclusion to question 2 . . ."
This reference to a previous question 2 in the only question
2 on the test is confusing to some students but most figure
out that it is a mistake. The student is never asked to formally
state the relationship between temperature and reaction rate.
This is taken into account in Criterion 3.
ME120B Rubric
Criterion 1: The student has completed at least part
of the data table and at least one of the following:
- Observations are written out for the experimental cases
that were completed. These may include ideas about why the
experiment did not work, color changes, calculations or
other relevant notes. (Data table) For example: 3rd time
- no change. I think we should have tried 150 ml of cold
water and 50 ml of hot. Maybe cold changes it.
- The student notes that they did not draw a previous conclusion
about the relationship between temperature and reaction
rate. (Question 2) For example: We did not draw a conclusion
in Question 2 - this is Question 2.
- The student notes that they could not draw a conclusion
about the relationship between temperature and reaction
rate because their experiment did not work. (Question 2)
For example: I do not know the relationship because we didn't
have time to do both hot and cold.
- The graph is properly labeled "time" and "temperature"
but incomplete because the experiment was not complete or
did not work. (Question 1)
OR
The student's completed graph is partially correct. (Question
1)
For example:
- The axes are labeled correctly but the points do not
correspond to the data.
- The points are correctly placed on the graph but the
axes and/or the scale are not labeled properly.
- The graph is very, very small - crammed into the corner
of the graph paper so that it is difficult to read.
- The "temperature" label is on the x axis and "time" is
on the y axis. The points are correctly placed according
to the student's data.
Criterion 2: The graph is correctly completed and labeled.
A line is drawn between the points. The point should correspond
to the data table. (Question 1)
For example (numbers are estimated):
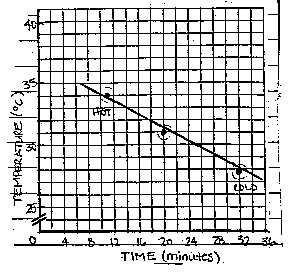
OR
If no data has been obtained, the student predicts what they
think might have occurred and draws a correct conclusion about
what the relationship between temperature and reaction rate
might be. (Question 2)
For example:
- If it had turned yellow, the time and temperature would
most likely have shown that a higher temperature requires
shorter time.
- The solution did not turn yellow, so we could not state
the conclusion, but I think that the hotter the water, the
faster the reaction.
Criterion 3: The student draws a conclusion about temperature
and reaction rate from experimental data. (Question 2) For example:
- From the graph you can see that if the temperature goes
down, the reaction takes longer.
- Generally, when the temperature is increased, the rate
of reaction is increased as well.
OR
The student lists or describes a situation from everyday life
that illustrates the temperature and reaction rate relationship.
The reaction referred to should be scientifically correct and
chemical in nature. (Question 2)
For example:
- The higher the temperate, the faster the reaction. One
place in everyday life where this can be seen is in cooking.
- To make a type of food melt more quickly, it is often
heated.
Not acceptable:
- An example in everyday life is when you run and your
body gets hot, your heart beats faster.
Criterion 4: The student applies the temperature and
reaction rate relationship to an everyday situation and correctly
describes what occurs at the molecular level. (Question 2)
For example:
- With reference to an ice cube. If the temperature is
raised the molecules begin to move faster, collide more
often, and consequently the ice melts. Thus as the temperature
rises, so does the rate of reaction.
OR
The student applies the temperature and reaction rate relationship
to an everyday situation and correctly describes in detail the
chemistry that occurs in the reaction. (Question 2) For example:
- Take for example, developing film. When developer is
added to film, it produces a chemical reaction that washes
off the silver bromide crystals. When the developer is warmer,
the reaction occurs more quickly.
|
|
|

