|
Contributed by: Council of Chief State School Officers (CCSSO)
ME 401: Matter and Its Transformation
Item #1
Item Description: (This portion may be completed
in a group.) Chemical elements are substances that cannot be made
into simpler substances by chemical means. Each element has properties
that distinguish it from other elements. Each element also has properties
that can be used to place it in a group with other elements
You are studying the elements in school. Your teacher gives you
samples of several elements and instructs you and your partners
to determine some properties of these elements. You will study each
element's magnetic property (how the element responds to the field
of a magnet) and density (the amount of mass in a given volume of
the element).
Individuals should answer the following: Based upon the experiments
[you complete], group the elements according to their relative magnetic
properties and their densities. Explain the basis for your classification
system.
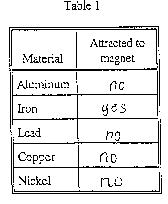
Content Definition: Magnetism is determined
in the following manner. Per the instructions given.
- Touch the material with the magnet. If the material is pulled
toward the magnet, write "yes" in the appropriate space in Table
1. If the material is not pulled toward the magnet, write "no"
in the appropriate space in Table 1.
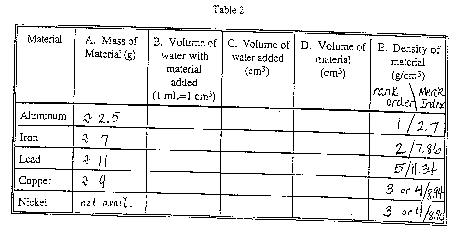
Density is determined in the following manner, per the instruction
given.
- Use the balance to find the weight of your sample. Record the
weight in Table 2, Column A;
- Add approximately 5.0 cm3 of water to the graduated
cylinder, and read the volume of water that you added to the nearest
0.1 cm3. Record the volume in Table 2, Column C. (Note:
1 ml is exactly 1 cm3; density is customarily reported
in g/cm3.);
- Complete submerge the metal in the water, being careful not
to spill any water;
- Read the new volume of the water to the nearest 0.1 cm3,
and record your result in Table 2, Column B;
- Subtract the original volume of water from the new volume of
water. The difference is the volume of the sample in cm3.
Record the difference in Table 2, Column D;
- Determine the density of your sample by dividing its mass by
its volume. Record the result in Table 2, Column E.
Scoring Parameters:
- Students will group the elements according to magnetic properties.
- Students will group the elements according to densities.
- Students will provide an explanation of the classification system.
Score Level Descriptions:
| NS |
Item is blank or response is irrelevant. |
| 1 |
The student uses her/his data to group elements
by magnetism or density. Logic cannot be followed in
explanation of grouping devised OR no explanation is
given. |
| 2 |
The student uses her/his data to group elements
by magnetism and density. Grouping and explanation exist,
however the logic or data supporting may be faulty. Magnetism
and density need not be related. |
| 3 |
The student demonstrates an adequate understanding
of magnetism and density, however shows only some ability
to relate the two to form an organizational system. Grouping
and explanation exist, but are weak and/or incomplete. Experimental
densities are derived correctly. |
| 4 |
The student demonstrates a full understanding
of magnetism, density, and the ability to relate the two to
form an organizational system. Student explanation and grouping
is easily understood and complete, citing specific examples
from their experiment or previous experience. Experimental densities
must be derived correctly. |
Note: Some "nickel" samples
are weakly magnetic, so response
will vary. |
ME 401: Matter and Its Transformations
Item #2
Item Description: On an exploration of the Appalachian
Mountains, suppose you find a solid material. When you break the material
apart, you see that the inside of the material is silvery and shiny.
Your teacher tells you that the shiny material consists of a single
element. Using your knowledge of the Periodic Table and of investigative
techniques, describe a procedure that you would use to classify and
identify the shiny material.
Content Definition: The substance is a solid.
It consists of one element, which is silvery and shiny. Possible
tests could include magnetism, water solubility, conductivity, flame
color change test, density, melting and boiling points, malleability,
ductility, and ability to reflect heat.
Scoring Parameters:
- Student describes some procedure to identify the element.
- Student explains how the results of specific tests will enable
the identification of the element.
- Student proposes some kind of step involving comparison to other
elements in the Periodic Table.
Note: Procedures does not equal tests.
Procedure is
more general; test more specific. |
Score Level Descriptions:
| NS |
Item is blank or response is irrelevant. |
| 1 |
The student shows a minimal understanding of possible
procedures identify the element. No tests are proposed. The
student is unable to clearly relate the identification of the
element to other elements in the Periodic Table. |
| 2 |
The student shows some understanding of possible
procedures to identify the element. A test is proposed, however
response does not clearly relate the results of the test to
the identification of the element, or to other elements in the
Periodic Table. |
| 3 |
The student shows adequate understanding of possible
procedures to identify the element. At least one procedure is
described in detail sufficient that the reader can visualize
and follow the procedure. The results of the test are described/interpreted
in such a way that the reader can understand their utility in
classifying or identifying a material. |
| 4 |
The student shows a full understanding of possible
procedures to identify the element. More than one procedure
is described in enough detail (steps, equipment) that the reader
can easily visualize and follow. The method of interpreting
results is explained for comparison of collected data to information
in the Periodic Table or to information about other elements. |
|



