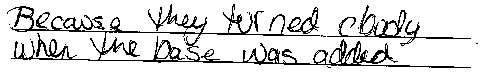Contributed by: New York State Education Department (NYSED)
Here are examples of possible score points. (You will notice that
the questions in these examples are not phrased exactly as they
were in the task although the basic idea is the same. It is apparent
that the questions were edited post-field testing.)
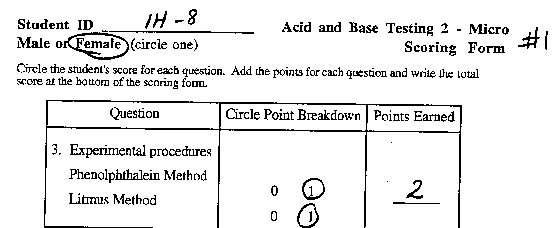
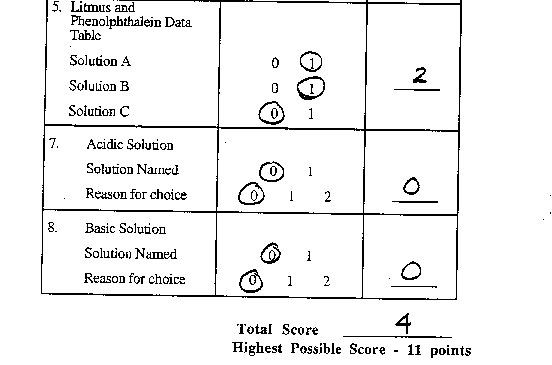
Task: At this station, you will experiment to determine
which of three solutions is acidic and which is basic.
Materials:
- disposable pipettes A - C
- chem plate marked A - C (3 rows)
- disposable pipette with phenolphthalein
- blue litmus paper
- red litmus paper
- safety goggles
- waste cup
- paper towels
- cassette case
Background:
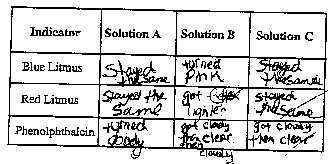
Phenolphthalein turns pink in a basic solution.
Blue litmus paper turns red (pink) when dipped
in an acidic solution.
Red litmus paper turns blue (purple) when dipped
in a basic solution. |
Directions:
- Put your safety goggles on.
- Think carefully about an experiment you could do to determine
which of the three solutions are acidic and which are basic.
- CARRY OUT YOUR EXPERIMENT.
- Record your observations in the data table below.
- Blot the wax paper with a paper towel and wipe off the test
card. Throw any garbage into the waste cup.
- In the space below, describe the procedures you followed in
conducting your experiment.

- Using the data you have collected and the background information,
which solution is acidic?
In the space below, explain the reason for your answer.

- Using the data you have collected and the background information,
which solution is basic?
In the space below, explain the reason for your answer.
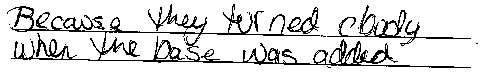
NYS Alternative Assessment in Science Project
Copyright, April 1996
The State University of New York
The State Education Department
Albany, New York 12234
Go to Next Student Work
|