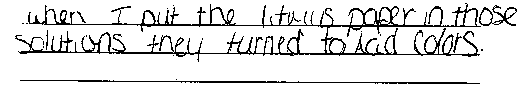|
Contributed by: New York State Education Department (NYSED)
Here are examples of possible score points.
Task: At this station, you will experiment to determine
which of three solutions is acidic and which is basic.
Materials:
- disposable pipettes A - C
- chem plate marked A - C (3 rows)
- disposable pipette with phenolphthalein
- blue litmus paper
- red litmus paper
- safety goggles
- waste cup
- paper towels
- cassette case
Background:
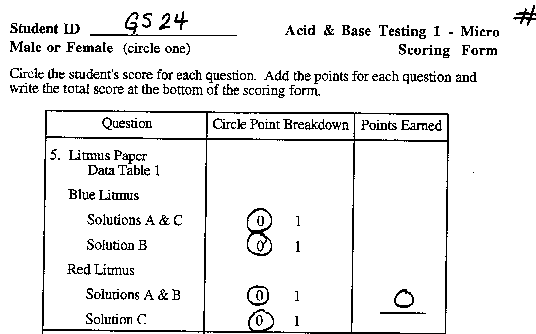
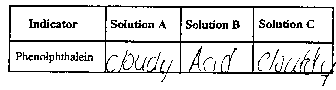
Phenolphthalein turns pink in a basic solution.
Blue litmus paper turns red (pink) when dipped
in an acidic solution.
Red litmus paper turns blue (purple) when dipped
in a basic solution. |
Directions:
- Put your safety goggles on.
- Place one drop of each solution on the circle with the same
letter in each of the three rows.
- Dip the end of a blue litmus paper into each of the three solutions
in row 1 and lay them on the plate.
- Immediately record the COLOR of the litmus paper on the
data table.
- Repeat steps 2 - 4 using the red litmus paper in the row
2 and lay them on the table.
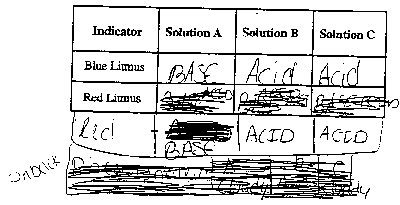
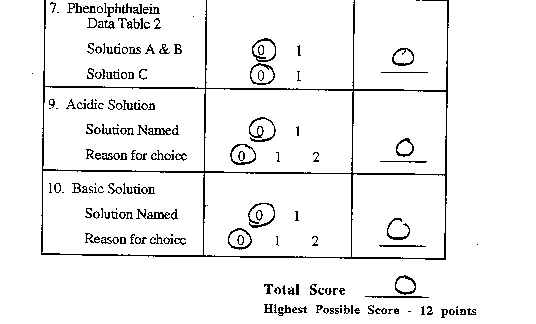
- Add one drop of phenolphthalein to each of the three solutions
in row 3.
- Record the COLOR of the phenolphthalein on the data table
below.
- Blot the chem plate with a paper towel. Throw any garbage into
the waste cup.
- Using the data you have collected and the background information,
which solution is acidic?
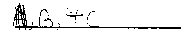
In the space below, explain the reason for your answer.
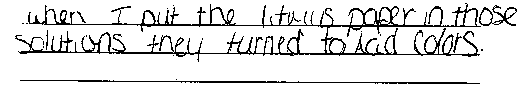
- Using the data you have collected and the background information,
which solution is basic?

In the space below, explain the reason for your answer.

NYS Alternative Assessment in Science Project
Copyright, April 1996
The State University of New York
The State Education Department
Albany, New York 12234
Go to Next Student Work
|