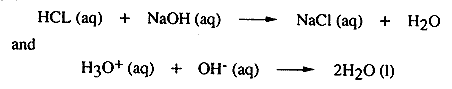|
 |
|
|
|
|
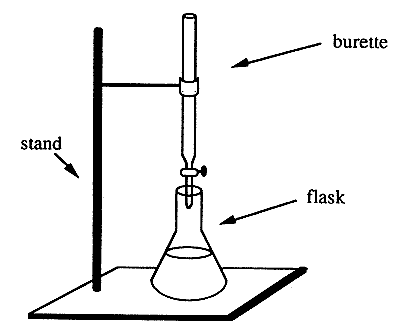
Grade 9-12 Performance Task IntroductionThis laboratory test presents a problem. Your task in Part A is to plan and design an experiment to solve the problem. You will have 30 minutes to complete Part A. At the end of 30 minutes, your answer sheet will be collected. You will then receive separate directions for Part B. In Part B you will use materials and equipment provided in the laboratory kit to collect experimental data for this problem. You may wish to do your preliminary planning on the sheet labelled "Working Copy." Write this plan on the appropriate answer sheet in your test booklet. Part AProblem Chemists are often required to determine the concentration of unknown acidic or basic solutions. You will be provided with an unknown solution that is either acidic or basic. Your problem is to design an experiment, using the materials (and/or others) listed below, to determine the concentration of the unknown solution provided. Express your concentration of the unknown material in moles of H+ (aq) per liter or moles of OH- (aq) per liter of solution. a) Under the heading PROCEDURE list in order the steps of the procedure you will use to solve the problem. You may include a diagram to help illustrate your plans for the experiment. Include any safety procedures you would follow. b) Construt a DATA TABLE or indicate any other method that you could use to record the observations and results that will be obtained. PLEASE NOTE: In Part A you are NOT to proceed with any part of the actual experiment. You are just to plan and organize a way to investigate the problem. Materials
ANSWER SHEET PART A - Experiment Design Organize your experiment design under the following
headings: PROCEDURE (Include diagram if appropriate)
DATA TABLE (For results and observations)
Part BYou will have 50 minutes to complete this part. You have been provided with a detailed Procedure (see next page) which you are to follow. Record your work for Part B on the answer sheet under the appropriate headings. a) Peform the experiment by following the steps outlined in the procedure. b) Under the heading RESULTS/OBSERVATIONS record the results of the experiment. Use statements, descriptive paragraphs, and tables of data where appropriate. c) Under the heading CALCULATIONS show all equations and calculations used. d) Under the heading CONCLUSION give an interpretation of your results. What did you learn from the experiment? e) You have also been provided with a sheet labelled "Working Copy." Use this scrap sheet for any initial calculations or conclusion. However, be sure to enter your final work on the appropriate answer sheets. f) At the end of 50 minutes, your answer sheets will be collected.
Materials
Procedure
ANSWER SHEET PART B - Experiment Report Organize your experiment report under the following
headings:
RESULTS/OBSERVATIONS Indicator: __________________________
CALCULATIONS
CONCLUSIONS
|