Contributed by: Council of Chief State School Officers (CCSSO)
TO THE STUDENT
Welcome to this experimental science exercise. We hope that you will
find it interesting and worthwhile. Carefully read through these directions
and the directions on the next page before you begin to work.
You may be part of a group for the first part of this exercise.
Each group should carry out the experiment and collect the data
together, but each student must record the data in his or her own
booklet. Be sure to record the data exactly as you observe them.
After the data has been collected, each student should answer the
questions independently.
After you have finished your experiment and have recorded all
of the data, you will be asked to answer some questions about the
experiment and the data you recorded. Your answers must be written
in this test booklet in the space provided. Make sure that you understand
each question before you begin to write. At any time while you are
writing your answers, you may look back to the directions for the
experiment and the data you collected. Be sure that your answers
are written as clearly and neatly as possible.
Before you turn the page, read the list of materials given below
and check to make sure that your group has everything listed.
Materials
- 3 opaque plastic cups
- 100 green beads
- 100 white beads
- colored pencils
|
|
AFTER YOU HAVE READ THE DIRECTIONS, TURN TO THE NEXT PAGE AND BEGIN.
Simulated Radioactive Decay
With a greater demand for alternative sources of energy, we have
turned toward using the energy released when radioactive atoms decay.
One problem with using nuclear energy is disposing of the nuclear
waste products. Spent fuel rods still contain some dangerous radioactive
atoms. These radioactive atoms will decay over time into non-radioactive
daughter atoms. This may take hundreds or even thousands of years.
Although it is impossible to predict when any individual atom
will decay, scientists are able to estimate the time it takes for
one-half of a sample of radioactive atoms to decay. This is called
the half-life. As an example, a radioactive form of iodine
(I-131) has a half-life of 8 days. This means that if you started
with one gram, after eight days about one-half of the sample would
have decayed into daughter atoms. The purpose of this activity is
to help you understand the concept of radioactive decay and half-life.
Since it is not possible to use radioactive atoms in this activity,
you will use a model system to simulate the process.
In this simulation the green beads will represent the radioactive
atoms and the white beads will represent the stable, non-radioactive
daughter atoms.
- Place 100 green beads in one cup and 100 white beads in a second
cup. Note the time on the clock and record this in Table 1.
- Take eight green beads out of the cup and replace them with
eight white beads. This will represent the decay process. Record
eight as the number of green beads removed in Sample 1 of your
data table. Place the green beads in the empty plastic cup so
you do not lose them.
- Cover and shake the cup containing the green and white beads
several times to mix them. Without looking, remove another
eight beads (this time you may be removing both green and white
beads). Count the number of green beads in this sample and record
this number under Sample 2 in the data table. Place the green
beads in the third cup. Return any white beads drawn to the cup
of mixed green and white beads. Replace the green beads you removed
from the cup with an equal number of white ones to maintain 100
beads in the mixing cup.
- Repeat Step 3 until you have taken a total of 50 green beads
from the mixing cup. Each time count and record the number of
green beads in the sample you removed from the mixing cup, removing
the green beads drawn and replacing them with white beads.
- Once your group has had 50 green beads "decay," note the time
and determine the elapsed time from when you started. Record this
in the data table.
- After you have taken the 50 green beads from the cup, separate
the green beads from the white beads. You should again place them
in two separate cups, one with 100 green beads and one with 100
white beads.
- Do the simulation over again, removing 4 beads per sample. Keep
removing beads until you have taken 50 or until you have taken
20 samples, whichever comes first. Follow the exact same mixing
and sampling procedure as you did before in Steps 2-6. Record
all values in the data table.
Table 1
| |
Sampling 8 at a time
|
Sampling 4 at a time
|
| Time simulation started: |
_____________________
|
____________________
|
| Time simulation ended: |
_____________________
|
_____________________
|
Elapsed time:
(approximately half-life value if no 50 beads are removed) |
_____________________
|
_____________________
|
|
| Sample
number |
Sampling 8 at a time |
Sampling 4 at a time |
|
Green beads remaining in cup
|
Green beads taken in this sample
|
Green beads remaining in cup
|
Green beads taken in this sample
|
|
0
|
100
|
___
|
100
|
___
|
|
1
|
|
|
|
|
|
2
|
|
|
|
|
|
3
|
|
|
|
|
|
4
|
|
|
|
|
|
5
|
|
|
|
|
|
6
|
|
|
|
|
|
7
|
|
|
|
|
|
8
|
|
|
|
|
|
9
|
|
|
|
|
|
10
|
|
|
|
|
|
11
|
|
|
|
|
|
12
|
|
|
|
|
|
13
|
|
|
|
|
|
14
|
|
|
|
|
|
15
|
|
|
|
|
|
16
|
|
|
|
|
|
17
|
|
|
|
|
|
18
|
|
|
|
|
|
19
|
|
|
|
|
|
20
|
|
|
|
|
Questions
Please answer the following questions by yourself.
- On the graph paper below, make a graph for each of your samples
showing the relationship between the number of green beads remaining
in the cup (y-axis) and the sample number (x-axis). Construct
both graphs using the same axes. Be sure to label your axes and
provide a legend. The graphs represent the decay curve for your
samples.
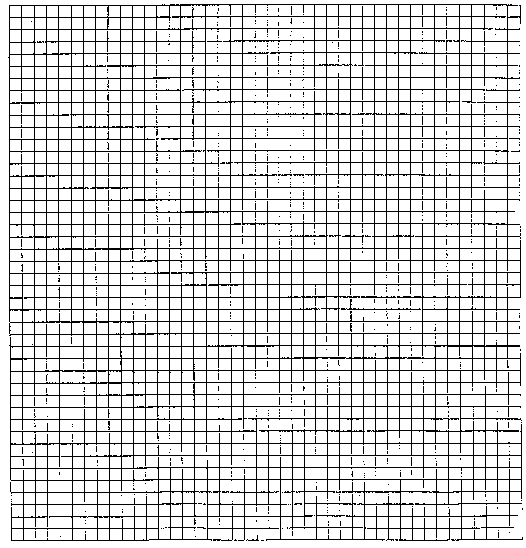
- Assume that one minute equals 100 years and that the sample
needs to decay to 1/16 of its original amount to be considered
"safe." You now have to safely dispose of your radioactive samples.
Choose one of your samples. Explain (1) how many years you would
have to be concerned about the radioactivity of the sample, and
(2) how you would dispose of this material. Be sure to justify
your responses.
________________________________________________________________________________________
________________________________________________________________________________________
________________________________________________________________________________________
________________________________________________________________________________________
________________________________________________________________________________________
________________________________________________________________________________________
________________________________________________________________________________________
________________________________________________________________________________________
________________________________________________________________________________________
________________________________________________________________________________________
________________________________________________________________________________________
________________________________________________________________________________________
|


