Contributed by: Council of Chief State School Officers (CCSSO)
Carefully read through these directions and the directions on
the next page before you begin to work.
You may be part of a group for the first part of this exercise.
If you are, the group should carry out the experiment and collect
the data together, but each student must record the data in his
or her own booklet. Be sure to record the data exactly as you observe
them. After the data have been collected, each student should answer
the questions independently.
After you have finished your experiment and have recorded all
of the data, you will be asked to answer some questions about the
experiment and the data you recorded. Your answers must be written
in this test booklet in the space provided. Make sure that you understand
each question before you begin to write. At any time while you are
writing your answers, you may look back at the directions for the
experiment and the data you collected. Be sure that your answers
are written as clearly and neatly as possible.
Before you begin the exercise, read the list of materials given
below and check to make sure that your group has everything listed.
Materials in the Kit
- cardboard hygrometer stand, Part A
- cardboard hygrometer stand, Part B
- one 5" piece of hollow shoestring (wick)
- 2 small o-rings (for holding wick on bulb)
- 2 thermometers with Fahrenheit scales
Materials Supplied by the School
- ice
- 50 ml of iced water
- water at room temperature
- narrow masking tape or Scotch tape
- 2 100 ml beakers or equivalent paper, styrofoam, or plastic
cups
- 1 pair of sharp, pointed scissors
- paper towels
- stopwatch or clock
|
Wet-Dry Bulb Hygrometers:
Measuring Relative Humidity and Apparent Temperature
The relative humidity of air compares the actual amount
of water vapor in the air at a given temperature to the amount of
water vapor that the air could hold at that temperature. Warm air
can hold more water vapor than cool air. The human body is cooled
in part by the evaporation of water from the skin, and the rate
of evaporation is affected by air's relative humidity. Air's apparent
temperature, a measure of how warm the air feels to a person,
also depends on the relative humidity of the air that is in contact
with the person's skin. Air with a high humidity has a higher apparent
temperature than air with a low humidity, because the more humid
air slows water's evaporation from the skin.
A hygrometer is a simple device that compares the air temperature
measured by a dry bulb thermometer
(a thermometer with a bare bulb) to that measured by a wet bulb
thermometer (a thermometer with a water-soaked wick attached to
the bulb). The temperature measured by the wet bulb thermometer
depends upon the rate of evaporation of the water from the wick,
which depends in turn upon the air's relative humidity.
In this event, you will assemble a simple hygrometer, and you
will use it to determine the relative humidity and apparent temperature
of the air in your classroom. Be sure to read all of the directions
before you begin this event.
Thermometer Calibration
- First, to correct for any defects in the thermometers, the
thermometers must be calibrated. Label the thermometers A and
B. Next, obtain some ice water from your teacher and add some
ice to it. There should be ice in the water at all times. Using
either thermometer, gently stir the ice and water mixture
for a few seconds. Leave both thermometers in the ice water and
wait 30 seconds. Then read the temperature of the iced water with
each thermometer. For either thermometer, if the temperature reading
is different from 32
 , find the correction
factor. The correction factor equals the thermometer's reading
minus 32 , find the correction
factor. The correction factor equals the thermometer's reading
minus 32 . If you get a negative correction
factor, record the factor with the minus sign. Record the correction
factors for Thermometers A and B in the spaces below. . If you get a negative correction
factor, record the factor with the minus sign. Record the correction
factors for Thermometers A and B in the spaces below.
Thermometer A correction factor = ____
Thermometer B correction factor = ____
- If a correction factor is positive for one of the thermometers,
whenever you measure a temperature with that thermometer, you
must subtract the correction factor from that measurement.
- If a correction factor is negative for one of the thermometers,
whenever you measure a temperature with that thermometer, you
must add the correction factor to that measurement.
- For example, suppose Thermometer A (or B) gives a reading of
34
 in the ice water. Then the correction
factor is 34 in the ice water. Then the correction
factor is 34 - 32 - 32 = +2
= +2 . Now, suppose the thermometer indicates
an air temperature of 70 . Now, suppose the thermometer indicates
an air temperature of 70 . Since the correction
factor for Thermometer A is positive, you must subtract
2 . Since the correction
factor for Thermometer A is positive, you must subtract
2 from this reading, so the air temperature
is really 68 from this reading, so the air temperature
is really 68 . .
- As another example, suppose Thermometer A (or B) gives a reading
of 30
 in the ice water. Then the correction
factor is -2 in the ice water. Then the correction
factor is -2 . Now, suppose the thermometer
indicates an air temperature of 70 . Now, suppose the thermometer
indicates an air temperature of 70 . Since
the correction factor is negative, you must add
2 . Since
the correction factor is negative, you must add
2 to this reading, so the air temperature
is really 72 to this reading, so the air temperature
is really 72 . .
- Discard the ice water.
(Note: (1) A proper calibration of these thermometers
would include corrections at several additional temperatures,
including one at the boiling point of water. Because of time
constraints, we are omitting these additional corrections. (2)
Each thermometer contains both Fahrenheit and Celsius scales.
Make sure you read only the Fahrenheit scale.)
Hygrometer Assembly
- Place Part A of the cardboard stand on a flat surface (table)
and fold it where indicated along the dotted lines, as shown in
Figure A.
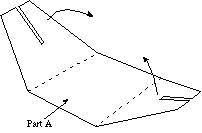
Figure A
- Pick up Part B and push out the 4 tabs with the holes. Then
assemble Parts A and B by fitting the slots of Part B into the
slots of Part A, as shown in Figure B.
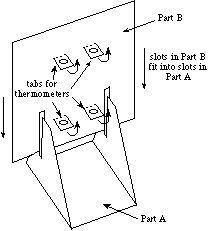
Figure B
- Push the two thermometers through the holes in the tabs, as
shown in Figure C1.
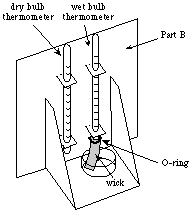 |
|
 |
|
Figure C1
|
|
Figure C2
|
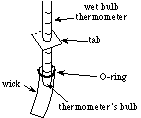
- Slide an o-ring onto one of the thermometers. (CAUTION:
Do not bend the thermometer, as the glass may break and cause
injury.)
- Slide the hollow shoestring wick over the bulb of the thermometer
that has the O-ring, making sure the bulb is completely covered,
as shown in Figure C2.
- To hold the wick in place, carefully roll the O-ring down over
the wick, so that the O-ring is above the bulb of the thermometer.
(The thermometer with the wick will be referred to as the wet
bulb thermometer, and the other thermometer will be referred
to as the dry bulb thermometer, as shown in Figure C1,
above.)
You are now ready to begin collecting your data.
- Obtain a cup of water at room temperature from your
teacher.
- Immerse the free end of the wick in the cup of water
at room temperature and wait 30 seconds, or until the wick is
saturated with water.
- Remove the cup of water and, using a small paper fan
or your hand, fan air over the wick and the bulb of the dry bulb
thermometer for about 15 seconds.
- Read to the nearest degree the temperatures shown on
the two thermometers. Correct the temperatures using the results
you obtained when you calibrated your thermometers (p. 2) and
record your corrected results in the row labeled 'Trial 1' in
Table 1.
- Subtract the corrected wet bulb temperature from the
corrected dry bulb temperature and record the temperature difference
in the row labeled 'Trial 1' in Table 1.
- For Trials 2 and 3, repeat Steps 3, 4, and 5. You will
average the results of Trials 1-3.
- To determine the relative humidity of the air in your
classroom: Look up the dry-bulb temperature and the temperature
difference that you recorded in Table 1 for Trial 1. Find your
dry-bulb temperature on the left side of Table 2, page 21. Find
your temperature difference on the top of Table 2. Using these
2 temperatures find the relative humidity for Trial 1 and record
the relative humidity in Table 1. Repeat Step 7 to obtain the
relative humidities for Trials 2 and 3.
- Calculate the average corrected dry bulb temperature
and the average relative humidity for the three trials (in Table
1), and record both averages in Table 1. In addition, write the
average corrected dry bulb temperature and the average relative
humidity above Table 3.
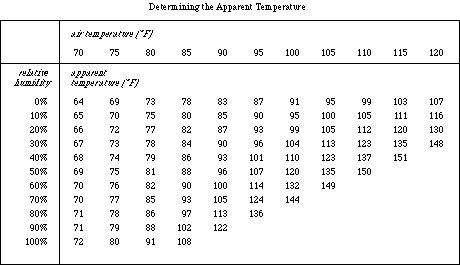
- To determine the apparent temperature of the air in
your room, use the averages from Step 8 in Table 3. Record the
apparent temperature in the space indicated below Table 1.
Table 1
|
Trial
|
Corrected Dry bulb temperature
( ) )
|
Corrected Wet bulb temperature
( ) )
|
Corrected Dry bulb temperature minus Corrected wet bulb
temperature ( ) )
|
Relative humidity (%)
|
1
|
|
|
|
|
2
|
|
|
|
|
3
|
|
|
|
|
Average
|
|
|
|
|
Apparent temperature ( ) of the air in your
classroom = ____ ) of the air in your
classroom = ____
Table 2
Relative Humidity (%)
Difference Between Dry-Bulb and Wet-Bulb Temperatures ( ) )
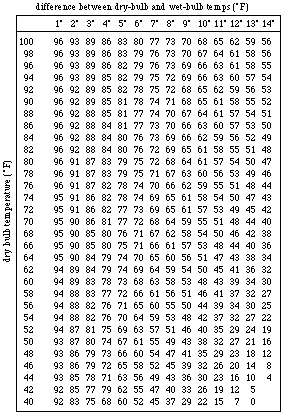
Table 3
Questions
Please answer the following questions by yourself.
- A thermometer that is used to measure the temperature
of a hot gas is calibrated in boiling water (the temperature of
the boiling point at an atmospheric pressure of 1 atm = 212
 ).
Use the following information to calculate the actual temperature
of the hot gas. Make sure to show all of your work. ).
Use the following information to calculate the actual temperature
of the hot gas. Make sure to show all of your work.
The temperature of the boiling water according to the thermometer
= 215 . .
The actual temperature of the boiling water = 212 . .
The temperature of the hot gas according to the thermometer
= 375 . .
The actual temperature of the hot gas = ____  . .
- The information in Table 4 was collected at noon each
day for 6 days at a given location. Based on the data in Table
4, estimate the noontime air pressure, percent cloud cover, and
precipitation at this location if the air temperature is 86
 and the relative humidity is 97%. Write your estimates in the
empty cells in Table 5. Explain how you arrived at your estimates.
and the relative humidity is 97%. Write your estimates in the
empty cells in Table 5. Explain how you arrived at your estimates.
Table 4
|
Day
|
Relative humidity (%)
|
Air temperature
( ) )
|
Air pressure (inches of mercury)
|
Cloud cover (%)
|
Amount of precipitation (inches)
|
|
1
|
6
|
75
|
28.95
|
40
|
0
|
|
2
|
98
|
85
|
28.30
|
100
|
1.5
|
|
3
|
30
|
77
|
29.01
|
25
|
0
|
|
4
|
40
|
75
|
29.50
|
0
|
0
|
|
5
|
55
|
78
|
29.20
|
30
|
0
|
|
6
|
60
|
79
|
28.95
|
50
|
trace
|
|
7
|
95
|
85
|
28.25
|
100
|
1.3
|
Table 5
|
Relative humidity (%)
|
Air temperature
( ) )
|
Air pressure (inches of mercury)
|
Cloud cover (%)
|
Amount of precipitation (inches)
|
97
|
86
|
|
|
|
|


 )
)





