 |
 |
|
|
|
|
Acids and Bases Alien - Form D Task: In this activity you will be working by yourself. You can write your answers directly on these pages. If you have a question, please raise your hand and we will come to help you. Please take the materials out of the bag in front of you. Put the materials on your placemat. Raise your hand if you are missing any of these materials: Materials:
All solutions are acids, bases, or neutral. You can use pH paper and a pH Color Chart to test whether a solution is an acid, a base, or neutral. Part 1: READING THE pH SCALE To practice using the pH paper:
1a. What is the color of the pH paper right after you dipped it into solution X? _____________ 1b. What number on the pH color chart goes with this color? _____________ 1c. Look at the chart below. Is solution X an acid, a base, or
neutral? _____________ 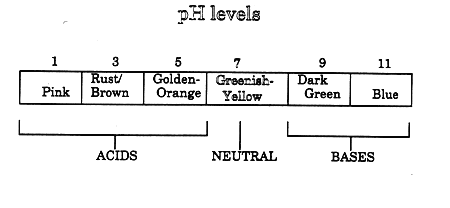
Part 2: THE PROBLEM You are an expert in blood chemistry. The blood of an alien creature is brought to your laboratory for analysis. The alien has a sever headache and a high temperature. The doctors say the alien has a sickness called acidosis-basicosis. This condition is caused by the blood being either too acidic (low pH) or too basic (high pH). The normal pH for alien blood is 7.0. The alien will die if its blood stays away from 7.0 for more than a few hours.
To find out what is wrong with the alien, test the pH of its blood
to see if it is too acidic or too basic.
2a. What was the color of the pH paper right after you took it out of the alien's blood? __________ 2b. What number on the pH color chart goes with this color? ___________ 2c. The alien is suffering from acidosis/basicosis because its blood is (circle the right choice):
Part 3: FINDING THE BEST MEDICINE The alien's spaceship has a first aid kit with three medicines. One or more of these medicines can cure acidosis/basicosis when it is added to the alien's blood. Your job is to figure out which medicine ( A, B, or C) will save the alien's life by bringing the pH of its blood back to normal. You should use the materials in front of you to conduct whatever experiments you think are needed to find out which medicine is best. Remember, always swirl a solution before you test it, and always use a new piece of pH paper for each test. 3a. Record the results of your experiment in the table below as you work.
3b. Write down and number the steps you followed as you conducted
your experiment. Be specific, so another student in your class could
conduct the experiment exactly as you did. For example, if you mixed
solutions together, tell how much of each solution you used. Part 4: DRAWING CONCLUSIONS Use the results of your experiment to answer the following questions: 4a. Which is the best medicine (A, B, or C) for the alien? ______________ 4b. Why is this medicine better than the other medicines? (You
may use a table, graph, or picture to help you explain your answer).
4c. How can a basic solution be turned into a neutral solution?
4d. How can an acidic solution be turned into a neutral solution?
Part 5: USING WHAT YOU LEARNED The people of Spring City were concerned because the fish in their pond were dying. They hired an environmental scientist who measured the pond's pH and found that it was too acidic. Pond fish need neutral water to survive. The people followed the specialist's advice and added Pro-Base, (a strong base) to the pond. After two days, the fish stopped dying. The people kept adding Pro-Base to the water and after three more days, the fish started dying again. In fact, the more Pro-Base they added, the more fish died. 
5a. Why did Pro-Base work at first, but not continue to work? 5b. What should the people in Spring City do now to save the fish in their pond? (Circle the best choice)
5c. Why did you choose this answer?
|