 |
 |
|
|
|
|
Contributed by: Connecticut Academic Performance Test (CAPT)
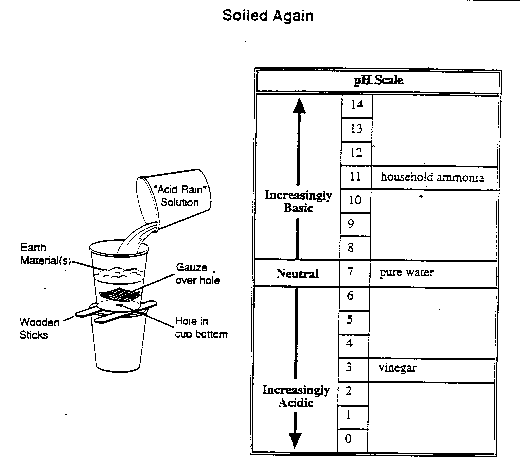 The results of the group's experiment are shown in the following table.
Question 1 - Event Score = 2
Question 2 - Event Score = 2
Question 3 - Event Score = 2
Question 4 - Event Score = 2
Previous Student Work | Next Student Work |